Henry Moseley was the first man who proved that every chemical element is determined by the number of protons it has, rather than by its mass. He reorganized Mendeleev’s Periodic System and noticed that there are four elements missing at the numbers 43, 61, 72, and 75.
These elements were technetium, promethium, hafnium, and rhenium, all discovered after Moseley died.

Henry Moseley was born in Dorset, in 1887, and was the son of Henry Nottidge Moseley, a biologist who taught anatomy and physiology at the University of Oxford. Both the father and son were characterized by their brilliance. Henry had been an outstanding student since his childhood and was awarded a King’s scholarship to attend Eton College where he won the prizes of chemistry and physics in 1906.
The same year he enrolled in his father’s alma mother, at Trinity College. After he graduated from Oxford in 1910 he started teaching physics at the University of Manchester and worked under the supervision of Ernest Rutherford. Three years later, Moseley had refused a fellowship offered by Rutherford and went back to Oxford where he was given laboratory facilities.

That same year, Moseley used the method of X-ray spectroscopy in physics and the Bragg’s diffraction law to measure the wavelength and frequencies of the resulting X-rays. He observed the elements and discovered that each one emits X-rays at a unique frequency. He also found a systematic mathematical relationship by plotting the square-root of X-ray frequency against elements’ atomic numbers. That has become known as Moseley’s law.
It had been an amazing discovery. More precisely, Moseley founding the basic difference between elements. And it was quite simple. Hydrogen has one proton. If we add one more, it becomes a completely different element – helium. One more proton makes it Lithium, and so on. This was a major discovery that happened in 1913 and largely contributed to science.
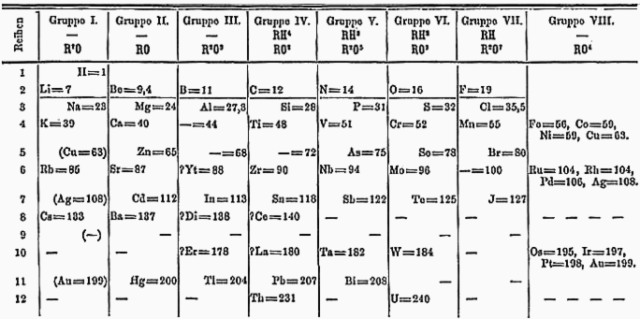
He also predicted that four elements were missing between Aluminium 13 and Gold 79, but at the time the existence of such elements was unknown. After he died, the radioactive synthetic elements technetium and promethium were discovered in 1937 and 1942.
The last two extremely rare and naturally-occurring stable elements, hafnium and rhenium were discovered in 1923 and 1925. Moseley gave a strong argument in terms of the exclusion of other gaps in the Period Table except for those 4 he found, and he was right.

With the outbreak of WWI in August 1914, Moseley enlisted with the Royal Engineers of the British Army and served as a technical officer in communications during the Battle of Gallipoli, in Turkey. While telephoning a military order, Moseley was shot by a Turkish sniper in April 1915.
The news of his death was received very sadly in the academic and scientific circles. Isaac Asimov has described Moseley’s death as “the most costly death of the War to mankind generally”. In the name of his ex-student, Ernest Rutherford campaigned and urged the British government to change its policy by preventing scientists from enlisting in the military service.
